Properties: Density and Viscosity
The properties outlines below are general properties of fluids which are of interest in engineering. The symbol usually used to represent the property is specified together with some typical values in SI units for common fluids. Values under specific conditions (temperature, pressure etc.) can be readily found in many reference books. The dimensions of each unit is also give in the MLT system (see later in the section on dimensional analysis for more details about dimensions.)
Density
- Mass Density
Mass Density,
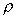 , is defined as the mass
of substance per unit volume.
, is defined as the mass
of substance per unit volume.
Units: Kilograms per cubic metre,
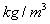 (or
(or
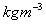 )
)
Dimensions:
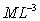
Typical values:
Water = 1000
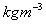 , Mercury = 13546
, Mercury = 13546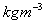 Air = 1.23
Air = 1.23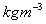 , Paraffin Oil = 800
, Paraffin Oil = 800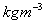 .
.
(at pressure =1.013
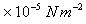 and Temperature =
288.15 K.)
and Temperature =
288.15 K.)
- Specific Weight
Specific Weight
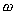 , (sometimes , and sometimes
known as specific gravity) is defined as the weight per
unit volume.
, (sometimes , and sometimes
known as specific gravity) is defined as the weight per
unit volume.
or
The force exerted by gravity, g, upon a unit volume of the substance.
The Relationship between g and
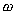 can be
determined by Newton's 2nd Law, since
can be
determined by Newton's 2nd Law, since
weight per unit volume = mass per unit volume g
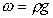
Units: Newton's per cubic metre,
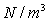 (or
(or 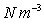 )
)
Dimensions:
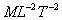 .
.
Typical values:
Water =9814
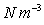 , Mercury = 132943
, Mercury = 132943 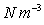 ,
Air =12.07
,
Air =12.07 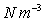 , Paraffin Oil =7851
, Paraffin Oil =7851 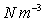
- Relative Density
Relative Density,
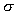 , is defined as the
ratio of mass density of a substance to some standard mass density.
, is defined as the
ratio of mass density of a substance to some standard mass density.
For solids and liquids this standard mass density is the maximum mass density for water (which occurs at
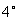 c)
at atmospheric pressure.
c)
at atmospheric pressure.
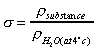
Units: None, since a ratio is a pure number.
Dimensions: 1.
Typical values: Water = 1, Mercury = 13.5, Paraffin Oil =0.8.
Viscosity
Viscosity, m, is the property of a fluid, due to cohesion and interaction between molecules, which offers resistance to sheer deformation. Different fluids deform at different rates under the same shear stress. Fluid with a high viscosity such as syrup, deforms more slowly than fluid with a low viscosity such as water.
All fluids are viscous, "Newtonian Fluids" obey the linear relationship
given by Newton's law of viscosity.
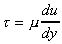 ,
which we saw earlier.
,
which we saw earlier.
where
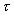 is the shear stress,
is the shear stress,
Units
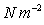 ;
; 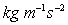
Dimensions
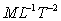 .
.
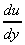 is the velocity gradient or rate of shear
strain, and has
is the velocity gradient or rate of shear
strain, and has
Units:
 ,
,
Dimensions
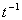
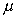 is the "coefficient of dynamic viscosity"
- see below.
is the "coefficient of dynamic viscosity"
- see below.
-
Coefficient of Dynamic Viscosity
The Coefficient of Dynamic Viscosity,
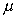 ,
is defined as the shear force, per unit area, (or shear stress
,
is defined as the shear force, per unit area, (or shear stress
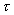 ), required to drag one layer of fluid
with unit velocity past another layer a unit distance away.
), required to drag one layer of fluid
with unit velocity past another layer a unit distance away.
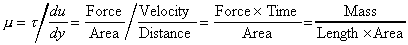
Units: Newton seconds per square metre,
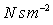 or
Kilograms per meter per second,
or
Kilograms per meter per second,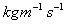 .
.(Although note that
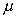 is often expressed
in Poise, P, where 10 P = 1
is often expressed
in Poise, P, where 10 P = 1 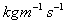 .)
.)Typical values: Water =1.14
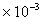
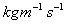 ,
Air =1.78
,
Air =1.78 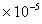
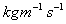 , Mercury
=1.552
, Mercury
=1.552 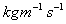 ,
,
Paraffin Oil =1.9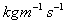 .
.
Kinematic Viscosity
Kinematic Viscosity,
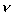 , is defined as the
ratio of dynamic viscosity to mass density.
, is defined as the
ratio of dynamic viscosity to mass density.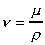
Units: square metres per second,
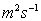
(Although note that n is often expressed in Stokes, St, where
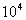 St = 1
St = 1 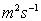 .)
.)
Dimensions:
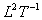 .
.
Typical values: Water =1.14
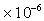
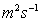 ,
Air =1.46
,
Air =1.46 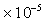
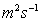 , Mercury
=1.145
, Mercury
=1.145 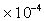
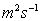 ,
,
Paraffin Oil =2.375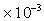
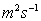 .
.
-
The density of a substance is the quantity of matter contained in a unit volume of the substance. It can be expressed in three different ways.
